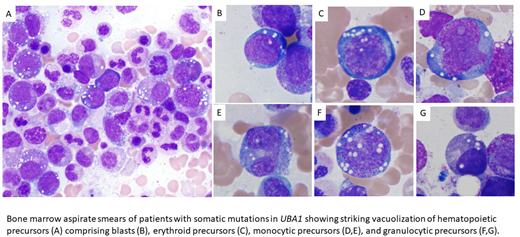
Somatic mutations in UBA1 in hematopoietic stem cells and myeloid cells have recently been described and are associated with adult-onset severe autoinflammatory diseases including relapsing polychondritis, Sweet syndrome, polyarteritis nodosa, and giant cell arteritis. This newly defined syndrome is named VEXAS (vacuoles, E1, X-linked, autoinflammatory, somatic). We performed clinicopathologic, cytogenetic, flow cytometric and molecular bone marrow assessment of 15 patients diagnosed with VEXAS and peripheral cytopenias. All patients were male, with a median age of 64y (IQR 45-80) and all had somatic missense mutations affecting the p.Met41 residue in UBA1 with variant allele frequencies (VAF) over 20% from peripheral blood or bone marrow samples and with lineage restriction to mature myeloid cells. Germline mutations at this residue are not reported in any public databases. Peripheral blood findings included macrocytic anemia in all patients (100%), thrombocytopenia in 9/15, and neutropenia in 1/15. All bone marrow aspirates (15/15; 100%) showed prominent vacuolization of both myeloid and erythroid precursors with few vacuoles noted in mature cells. Of the 9 patients tested for serum copper levels all were normal or borderline low. Marrow was hypercellular with granulocytic and megakaryocytic hyperplasia in 12/15 (80%). Definitive WHO criteria for MDS was met in 5/15 (40%) with 3 cases of MDS-MLD, and 2 of MDS-SLD. The remaining cases were suspicious for MDS with low or borderline levels of dyspoiesis that did not exceed greater than 10% of cells in respective lineages meeting criteria for clonal cytopenia of undetermined significance (CCUS). Of the MDS cases, dysplasia was seen in megakaryocytes (5/5), myeloid (2/5) and erythroid (1/5) precursors. Cytogenetic abnormalities were detected in 2/5 MDS patients involving t(3;12)(q21;q13) in one case, and del (5q)/del (13q) in another case. Next generation sequencing analyses were performed in 9/15 patients and showed mutations in DNMT3A in 2/5 MDS cases (VAF 43% and 44%), CSF1R in 1/5 MDS (VAF 3.1%), GNA11 in 1/5 MDS (VAF3.3%) and EZH2 mutation (VAF 22%) in one of the patients without overt MDS. Clonal plasma cell or B-cell populations were diagnosed in 6/15 patients with 3 plasma cell dyscrasias, and 2 cases of monoclonal B-cell lymphocytosis. The most common abnormalities detected by bone marrow flow cytometry analysis were severely reduced or absent B-cell precursors, inverted CD4:CD8 ratios and abnormal expression of CD56 on monocytes.
In summary, hematologic disorders were identified in 10/15 VEXAS patients, including both myeloid and lymphoid clonal disease comprising MDS, plasma cell dyscrasias, and monoclonal B cell lymphocytosis. Based on the presence of UBA1 somatic mutations in hematopoietic cells of all VEXAS patients, the patients without evidence of overt neoplasia in this study met criteria for CCUS. Importantly, all patients had characteristic vacuoles in myeloid and erythroid precursors in the marrow without evidence of copper deficiency. These findings suggest that vacuolization of hematopoietic precursors in marrow of cytopenic patients, particularly in male patients and in the setting of systemic inflammation and normal copper levels, should prompt evaluation for somatic UBA1 mutations. In addition to severe autoinflammatory disease manifestations, VEXAS patients appear to confer increased risk for development of both myeloid and lymphoid/plasma cell neoplasia and require surveillance for disease progression.
Young:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.